Abstract
Introduction
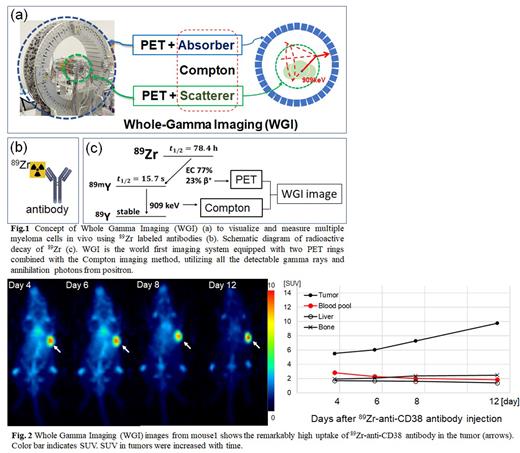
Visualization and measurement of multiple myeloma (MM) cells in vivo by means of positron emission tomography (PET) is expected to determine biopsy sites, personalize treatments, and make an early diagnosis of recurrence. The positron radioisotope 89Zr is one of the promising radionuclides for PET image due to the long radioactive half-life of 3 days and stable labelling of antibodies. 89Zr also emits 909 keV single gamma-rays about 4 times more frequently than positrons, causing just noise to make PET images. To overcome this disadvantage of 89Zr, we propose a new imaging method that detects and uses the single gamma-rays as well as positrons for imaging, called whole gamma imaging (WGI). WGI is composed of PET and Compton imaging methods, equipped with two PET detector rings; both of the inner ring and the outer ring also acts as a scatterer and an absorber, respectively, for Compton imaging method (Fig. 1).
In this study, we have firstly validated the affinity and specificity of antibodies recognizing CD38 and SLAMF7 in two types of human MM cell lines: RPMI8226 with CD38 expression and U226 with CD38 and SLAMF7. Then we carried out imaging in xenograft model mice with WGI using an 89Zr-antibody tracer and finally compared the quantitative values by WGI to those by ex-vivo biodistribution method.
Methods
Specific binding of antibodies was evaluated up to 10 × 106 cells of each MM cell type. An inhibition assay was performed using unlabeled IgG. 89Zr was produced in a cyclotron and neutralized 89Zr-oxalate was added to chelate-conjugated antibodies. Two severe combined immunodeficiency (SICD) mice after 3 Gy gamma-ray irradiation were implanted subcutaneously in the right flank with 3×107 MM cells in 100μL of PBS. On 12 days after the implantation, the tumor volume reached approximately 180 mm3 (mouse 1) and 110 mm3 (mouse 2). Based on the in-vitro study, 89Zr-anti-CD38 antibodies at the dose of 3.3 MBq were injected via a tail-vein. WGI imaging was performed on 4, 6, 8, and 12 days after the tracer injection. After WGI imaging on day 12, mice were euthanized for ex-vivo biodistribution analysis; blood was collected via cardiac puncture into counting tubes. Tumor and normal tissues: lung, liver, spleen, kidney, muscle, heart, bone (tibia), pancreas, stomach, intestine, brain, and lymph node were excised, and their weight and radioactivity were measured, and %ID/g values were calculated.
Results
High specific binding fractions of anti-CD38 antibodies were observed for RPMI8226 cells, reaching to approximately 50% at 10 × 106 cells; while only 5% for U226 cells. Those of anti-SLAMF7 antibodies were under 1% for both types of tumor cells. The inhibition assay using cold IgG blocked the binding of anti-CD 38 antibodies (Kd = 1.2 nM) and chelate-conjugated anti-CD38 antibodies (Kd = 1.4 nM) to RPMI8226. Then we decided to use 89Zr-anti-CD38 antibody and RPMI8226 cells for the WGI imaging study.
WIG imaging showed that 89Zr-anti-CD38 antibody uptake in tumors increased over time, being up to standardized uptake value (SUV) 9.8 on 12 days after tracer injection. The uptake in the healthy tissues gradually decreased with time except for the bone (Fig. 2).
Ex-vivo biodistribution revealed that the highest 89Zr-anti-CD38 antibody uptake in tumors was 92.4%ID/g (SUV, 18.5) and 97.5%ID/g (SUV, 19.5) in mice 1 and 2, respectively. That in healthy tissues was under 27.7%ID/g. Considering the specific activity of 89Zr-anti-CD38 antibodies of 4.2 MBq/nmol, 89Zr-anti-CD38 antibodies accumulated in RPMI8226 tumors at approximately 730 fmol/mg.
Conclusion
We proposed a new imaging method, called WGI, which clearly visualized MM cells and was able to estimate the uptake values of 89Zr-anti-CD38 antibody binding to specific antigen in MM tumors.
Disclosures
Ishibashi:Takeda Pharma.: Speakers Bureau; GlaxoSmithKline Japan: Research Funding. Imai:Takeda Pharma: Research Funding, Speakers Bureau; Bristol Myers Squibb: Research Funding, Speakers Bureau; Janssen Pharmaceutical K.K.: Speakers Bureau; Sanofi: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal